X-chromosome inactivation is a fascinating biological mechanism that ensures females, who possess two X chromosomes, do not express twice the number of X-linked genes compared to males, who carry only one. This intricate process is pivotal for maintaining genetic balance and plays a crucial role in the development of certain genetic disorders, such as Fragile X Syndrome and Rett Syndrome. Research led by renowned scientist Jeannie T. Lee at Harvard Medical School has shed light on the mechanics of this chromosomal silencing, revealing novel insights that could pave the way for potential therapies. By understanding how X-chromosome inactivation operates, scientists hope to unlock treatments for conditions linked to mutations on the X chromosome, thus alleviating the burden of these disorders. The implications of this research extend beyond basic science, offering hope to thousands affected by these challenging genetic diseases.
The phenomenon known as X-chromosome inactivation, or lyonization, serves as a critical biological process in female mammals, balancing the expression of X-linked genes in the face of genetic complexity. Through chromosomal silencing, one X chromosome is effectively rendered inactive, allowing for a stable genetic environment that is essential for normal development. This process has significant implications in the context of genetic conditions like Fragile X and Rett syndromes, where mutations on the X chromosome lead to severe health challenges. Insights from researchers such as Jeannie T. Lee have advanced our understanding of this mechanism, linking it to innovative therapeutic strategies for reactivating beneficial genes that can mitigate the effects of these disorders. By exploring the dynamics of X-linked gene expression, scientists are opening new avenues for clinical applications that could revolutionize treatment for affected individuals.
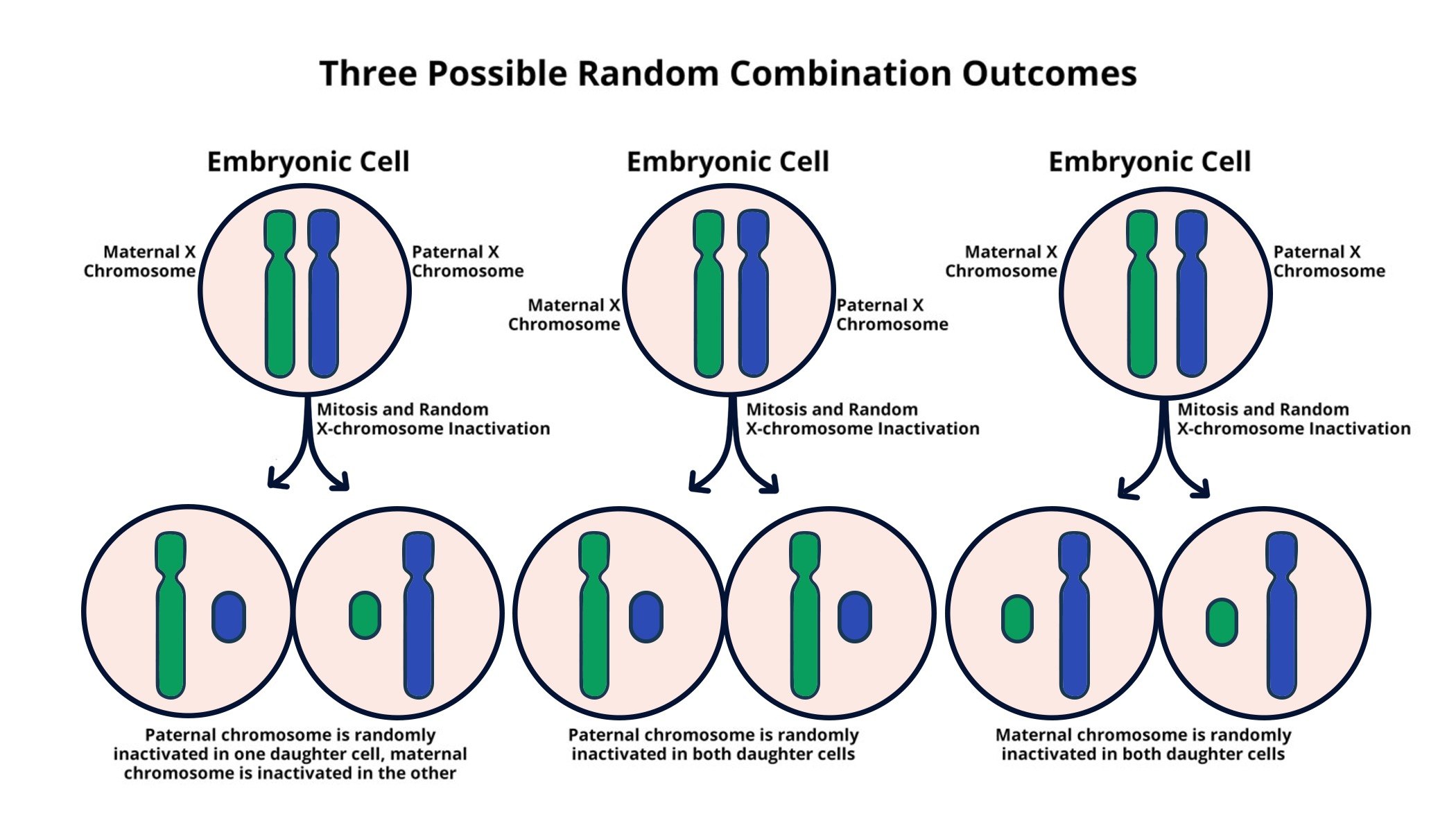
Understanding X-Chromosome Inactivation Mechanisms
X-chromosome inactivation (XCI) is a crucial biological process that ensures dosage compensation for genes located on the X chromosome in females. Unlike males, who carry a single X chromosome, females possess two, creating a risk of gene overexpression. To resolve this, one of the X chromosomes in each cell undergoes inactivation, effectively shutting down its gene activity. The mechanism behind XCI remained elusive for years, but research led by prominent geneticists, like Jeannie T. Lee at Harvard Medical School, has shed light on its complexity. Lee’s work has revealed that a specialized RNA molecule, Xist, plays a pivotal role in modifying the chromosomal environment, paving the way for a better understanding of this vital process and its implications for genetic disorders like Fragile X Syndrome and Rett Syndrome.
The concept of X-chromosome inactivation challenges our understanding of genetic expression and chromosomal dynamics. Lee’s recent findings illustrate how a gelatinous substance, reminiscent of Jell-O, envelops chromosomes, providing both structural support and functionality. This unique substance allows molecules like Xist to interact with the chromosomal environment, effectively silencing one of the X chromosomes. By gaining insights into these mechanisms, researchers aim to develop innovative therapeutic strategies that may reactivate the inactivated X chromosome, providing potential treatments for conditions primarily affecting females, including Rett Syndrome, characterized by severe neurodevelopmental deficits.
Implications for Fragile X Syndrome and Rett Syndrome
The elucidation of X-chromosome inactivation mechanisms holds transformative potential for addressing Fragile X Syndrome and Rett Syndrome. Fragile X Syndrome, a genetic condition caused by mutations on the X chromosome, leads to significant cognitive impairment and emotional challenges. Meanwhile, Rett Syndrome presents severe developmental issues predominantly in females, owing to mutations affecting X-linked genes. By reactivating the healthy gene residing on an inactivated X chromosome, researchers led by Jeannie T. Lee believe they could offer genuine hope to individuals afflicted by these disorders. The potential to restore normal gene function promises to alleviate symptoms and significantly improve quality of life for affected individuals.
The journey from understanding X-chromosome inactivation to potential treatments for genetic disorders embodies the essence of translational medicine. Lee’s research team has devised innovative methods to intentionally reactivate X-linked genes, a breakthrough that may lead to therapies for both Fragile X and Rett Syndromes. As they continue to optimize these techniques, the focus remains not only on efficacy but also on safety, ensuring that any therapeutic interventions will not adversely impact relevant teratogenic genes. The findings herald a new era in genetic disorder treatment, moving from fundamental research to impactful clinical applications.
Exploring the Mysteries of Chromosomal Silencing and Reactivation Strategies
Despite significant progress in understanding X-chromosome inactivation, several enigmas remain surrounding the genetic silencing process. Researchers have noted that while certain mutated genes can be reactivated without adversely affecting the healthy counterparts, the underlying mechanisms of this selectivity are still poorly comprehended. This phenomenon raises intriguing questions about the capacity of cellular resources and the interplay between different gene functions on the X chromosome. Jeannie T. Lee’s lab continues to investigate why only certain mutated genes are targeted for activation while healthy genes remain safeguarded from loss of function.
Lee’s research highlights the intricacies of chromosomal dynamics, emphasizing that cells possess limitations in gene utilization that could explain these events. By refining our grasp of how specific genes are managed within the chromosomal environment, scientists can leverage this knowledge to develop therapies that not only focus on genetic reactivation but also consider the complex interplay of other genes. Understanding these interactions could lead to more effective interventions with fewer complications, ultimately optimizing treatment strategies for various genetic disorders linked to the X chromosome.
Jeannie T. Lee’s Revolutionary Research in Genetics
Jeannie T. Lee has emerged as a leading figure in the field of genetics, particularly for her groundbreaking work on X-chromosome inactivation. Her innovative approach combines fundamental research with practical applications, bridging the gap between scientific inquiry and tangible medical solutions. For decades, her lab has been probing the mechanisms of chromosome silencing, aiming to unravel the complexities of how genes are activated or inactivated. Lee’s dedication to understanding these processes is evident in her commitment to conducting thorough investigations and utilizing advanced techniques of molecular biology.
The transformative potential of Lee’s research lies not only in elucidating the biological underpinnings of genetic disorders but also in paving the way for innovative therapies. As her team develops novel strategies to reactivate inactivated X-linked genes, they bring hope to millions affected by X-chromosome-linked conditions. Lee’s vision extends beyond simple scientific achievement; it embodies the desire to translate rigorous research into real-world interventions that could change lives. This synergy of knowledge and application positions Lee and her team at the forefront of genetic research and therapeutic development.
The Future of Gene Therapy and Clinical Trials
As the landscape of gene therapy continues to evolve, the work of researchers like Jeannie T. Lee heralds a new dawn in treatment possibilities for genetic disorders. Following successful laboratory findings, the next critical step involves the transition from research to clinical application. Lee’s team is currently engaged in refining their therapeutic methods and ensuring robust safety measures are in place before moving forward into clinical trials. The dream of translating lab discoveries into effective treatments for conditions like Fragile X Syndrome and Rett Syndrome is becoming more tangible, with expectations set for breakthroughs in the coming years.
The push towards clinical trials represents a pivotal moment in genetic medicine, as research translates to potential solutions for individuals suffering from life-altering disorders. Lee’s focus on safety, efficacy, and specificity in her research follows a meticulous design, which is crucial in the sensitive landscape of gene therapy where adverse outcomes could have significant implications. As her and her colleagues proceed through the phases of testing and evaluation, the medical community remains hopeful that these advancements will yield life-changing interventions for many patients.
Navigating the Challenges of Genetic Disorders
While the progress made in understanding X-chromosome inactivation and its implications for genetic disorders is remarkable, significant challenges remain in translating these findings into practical therapies. For conditions such as Fragile X Syndrome and Rett Syndrome, the complexity of the human genome presents hurdles for treatment development. Each disorder involves unique mutations and manifestations which require tailored approaches. Lee’s research focuses on overcoming these challenges by utilizing cutting-edge genetic engineering techniques to reactivate healthy genes, but the variability of genetic expression adds layers of difficulty.
Moreover, understanding the biological nuances of chromosomal silencing is crucial not only for developing targeted therapies but also for ensuring those treatments are safe. Researchers must navigate ethical considerations alongside scientific pursuits, carefully assessing the risks and benefits of reactivating specific genes. By addressing these multifaceted challenges, the scientific community can position itself to develop effective methodologies that not only treat symptoms but also target the underlying causes of genetic disorders, potentially transforming patient care.
Unveiling Patient-Centric Agendas in Genetic Research
At the heart of Jeannie T. Lee’s research agenda is a profound commitment to patient-centric approaches in genetic research. For those living with disorders caused by mutations on the X chromosome, the progression towards effective treatments is not just a scientific endeavor; it is a human endeavor. As Lee and her colleagues embark on clinical trials, their focus extends beyond lab work to the people their discoveries will impact. This perspective emphasizes the urgency and importance of fostering strong collaborations between researchers, clinicians, and patient communities.
Throughout the journey from bench to bedside, prioritizing the needs and insights of patients enriches the research process. This holistic approach not only enhances the relevance of genetic studies but also fosters a sense of community among stakeholders. By actively engaging with individuals and families affected by Fragile X Syndrome and Rett Syndrome, researchers like Lee adopt a collaborative model that respects the experiences of patients, ultimately driving the development of interventions that are not only scientifically sound but also deeply meaningful and beneficial.
Long-Term Outlook for Genetic Intervention Strategies
The long-term outlook for genetic intervention strategies, particularly in the context of X-chromosome inactivation, is optimistic thanks to influential researchers like Jeannie T. Lee. As the understanding of genetic disorders expands, so too does the potential for developing safe and effective treatments. Lee and her team’s commitment to refining methods to reactivate X-linked genes indicates that the future holds promise for those afflicted by disorders like Fragile X Syndrome and Rett Syndrome. The translational capabilities emerging from their work could revolutionize how these conditions are treated, providing hope for families affected by these challenges.
Equally important is the emphasis on continuous research and the importance of long-term data collection during clinical trials. This informs the scientific community not only about the efficacy of potential treatments but also about the timelines for expected outcomes and the sustainability of such interventions. With a focus on improvements and adjustments based on real-world patient experiences, researchers can adapt their methodologies, ensuring the development of robust gene therapies that will be transformative in the lives of many.
Advancing Knowledge Through Collaborative Research
The collaborative spirit embedded within Jeannie T. Lee’s research underscores the importance of teamwork in advancing genetic understanding and treatment methodologies. By bringing together diverse experts from various fields, her lab exemplifies the benefits of interdisciplinary collaboration in addressing complex genetic issues. The partnership among geneticists, biologists, and clinical researchers fosters a rich environment for innovation, where knowledge-sharing accelerates discovery and application in the real world.
This collaborative framework is crucial, particularly given the multifaceted nature of genetic disorders that benefit from a variety of perspectives. As researchers across disciplines unite their expertise, they can devise more comprehensive solutions that tackle the intricacies of disorders like Fragile X Syndrome and Rett Syndrome. Looking ahead, these cooperative efforts will likely pave the way for revolutionary advancements that integrate innovative science with meaningful patient outcomes, heralding a new era in the treatment of genetic diseases.
Frequently Asked Questions
What is X-chromosome inactivation and why is it important for females?
X-chromosome inactivation is a biological process that occurs in female mammals, where one of the two X chromosomes is randomly silenced to ensure that females do not produce double the amount of proteins encoded by genes on the X chromosome. This process is vital for maintaining gene dosage balance and preventing abnormal gene expression that could lead to genetic disorders.
How does X-chromosome inactivation relate to Fragile X Syndrome?
Fragile X Syndrome is caused by mutations on the X chromosome. Since females have two X chromosomes, X-chromosome inactivation can mask the effects of a mutated gene by using the healthy version on the other X chromosome. Understanding X-chromosome inactivation is crucial for developing potential therapies for Fragile X Syndrome by potentially reactivating silenced healthy genes.
What role does Jeannie T. Lee play in the study of X-chromosome inactivation?
Jeannie T. Lee is a prominent researcher at Harvard Medical School who focuses on understanding the mechanisms behind X-chromosome inactivation. Her lab’s recent discoveries regarding the biophysical properties of the chromosomal silencing process may pave the way for new treatments for X-linked genetic disorders, including Fragile X Syndrome and Rett Syndrome.
Can X-chromosome inactivation provide answers for therapies related to Rett Syndrome?
Yes, understanding X-chromosome inactivation may help researchers develop targeted therapies for Rett Syndrome. Since this disorder is also linked to mutations on the X chromosome, insights from the inactivation process could lead to innovative strategies to reactivate silenced healthy genes that could alleviate symptoms of Rett Syndrome.
What is the significance of the ‘Jell-O’ substance in X-chromosome inactivation?
The ‘Jell-O’ substance surrounding chromosomes, as described by Jeannie T. Lee, plays a crucial role in X-chromosome inactivation. It helps maintain the structure of chromosomes and affects how RNA molecules like Xist can interact with the chromosome, leading to the successful silencing of one X chromosome and ensuring proper gene expression.
What future research directions are being pursued regarding X-chromosome inactivation and genetic disorders?
Future research will focus on refining methods to reactivate silenced X-linked genes in cells, with the goal of developing clinical therapies for genetic disorders such as Fragile X Syndrome and Rett Syndrome. Jeannie T. Lee’s lab is continuing to investigate the underlying mechanisms of this process, aiming to translate these findings into potential treatments that minimize side effects.
Is X-chromosome inactivation relevant for male genetic disorders?
While males only have one X chromosome and do not undergo X-chromosome inactivation, understanding this process is still relevant. Certain mutations on the X chromosome can lead to disorders like Fragile X Syndrome, and insights gained from studying X-inactivation could inform strategies to address these mutations effectively, even in male patients.
What are the challenges in understanding X-chromosome inactivation?
Despite recent advancements, understanding the complete mechanics of X-chromosome inactivation remains challenging. Key questions include how the process selectively reactivates mutated genes while sparing healthy ones, and further research is needed to elucidate these intricate interactions to develop effective treatments for associated genetic disorders.
| Key Aspect | Explanation |
|---|---|
| X-Chromosome Characteristics | Females have two X chromosomes, whereas males only have one. This leads to the need for one X chromosome to be inactivated in females. |
| Inactivation Mechanism | The gene Xist on the X chromosome produces an RNA molecule, which alters the properties of a gelatinous substance surrounding the chromosome, leading to its inactivation. |
| Potential Therapies | Reactivate inactivated X-linked genes could treat disorders like Fragile X Syndrome and Rett Syndrome. |
| Research Significance | The study provides insights that could lead to breakthroughs in treating genetic disorders by unsealing inactivated X chromosomes. |
| Future Directions | Further refinements and safety studies will lead to clinical trials for potential therapies. |
Summary
X-chromosome inactivation is a critical biological process that allows females to manage the presence of two X chromosomes. Understanding this process, as explored in the recent research by Jeannie T. Lee’s lab, opens doors to potential therapeutic avenues for genetic disorders associated with the X chromosome, such as Fragile X and Rett syndromes. This research not only clarifies the mechanism by which one X chromosome is silenced but suggests innovative treatments that could reactivate the healthy gene within the inactivated chromosome, promising significant advancements in genetic medicine.